Nitrous Oxide: Revealing Its Entities
The chemical formula for nitrous oxide, also known as “laughing gas,” is N2O. It is an odorless, colorless gas. Despite having a humorous moniker, nitrous oxide has a complex past, a wide range of uses, and more recently, concerns about misuse. The characteristics, applications, and possible dangers of nitrous oxide are examined in this article.
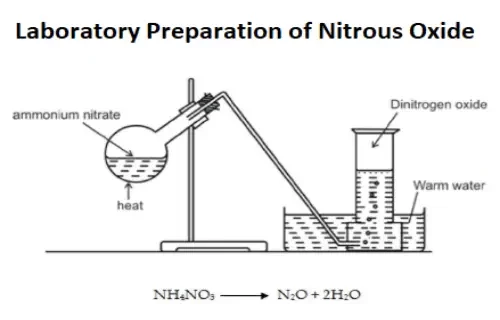
Properties and Production
Nitrous oxide is a diatomic molecule composed of two nitrogen (N) atoms and one oxygen (O) atom. It is produced through the thermal decomposition of ammonium nitrate, a common method used in industrial settings. The gas itself is non-flammable and has a slightly sweet taste.
Explore More Features

Medical Applications
Since the late 18th century, nitrous oxide has been used as an anesthetic in a variety of medical and dental treatments. It is especially prized for its analgesic and anxiolytic qualities, which help patients feel less anxious and relieve pain. Nitrous oxide is frequently given in conjunction with oxygen and is thought to be safe when used by qualified medical personnel in controlled settings.
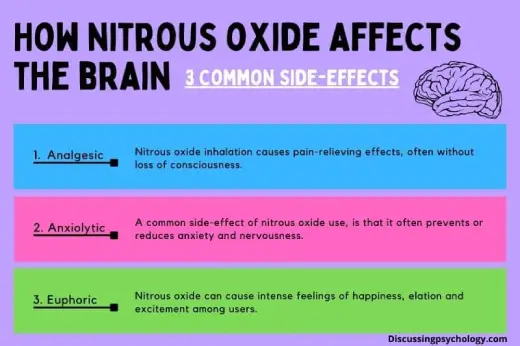
Recreational Use and Its Dangers
Nitrous oxide has become more well-liked as a recreational drug outside of its medicinal uses, particularly in the music and festival scenes. In an attempt to achieve the fleeting exhilaration and changed perception that the gas can produce, users frequently inhale it from tiny canisters or balloons.
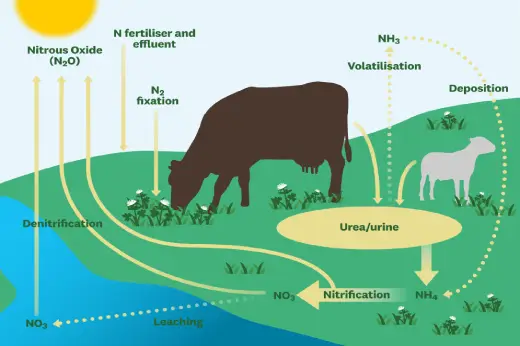
Environmental Impact
Another important greenhouse gas that contributes to climate change is nitrous oxide. Compared to other greenhouse gases, its concentration in the atmosphere is comparatively low, but its potency is significantly higher. In an effort to lessen emissions from industrial sources and address its part in climate change, efforts are being made.
Conclusion
Nitrous oxide is an intriguing molecule with a complex history, serving as both a vital medical tool and a recreational drug. The increasing popularity of recreational use of marijuana highlights the need for public education about the risks involved, even as its medical applications continue to improve patient care. To maximize its advantages and minimize potential harm to people and the environment, nitrous oxide must be used responsibly in medical contexts while also being prevented from being misused.